Fundamentals of Hydraulic Engineering Systems 4th Edition Houghtalen Solutions Manual
$26.50$50.00 (-47%)
Fundamentals of Hydraulic Engineering Systems 4th Edition Houghtalen Solutions Manual.
You may also like
This is completed downloadable of Fundamentals of Hydraulic Engineering Systems 4th Edition Houghtalen Solutions Manual

Product Details:
- ISBN-10 : 0136016383
- ISBN-13 : 978-0136016380
- Author:
Fundamentals of Hydraulic Engineering Systems, Fourth Edition is a very useful reference for practicing engineers who want to review basic principles and their applications in hydraulic engineering systems. This fundamental treatment of engineering hydraulics balances theory with practical design solutions to common engineering problems. The author examines the most common topics in hydraulics, including hydrostatics, pipe flow, pipelines, pipe networks, pumps, open channel flow, hydraulic structures, water measurement devices, and hydraulic similitude and model studies. Chapters dedicated to groundwater, deterministic hydrology, and statistical hydrology make this text ideal for courses designed to cover hydraulics and hydrology in one semester.
Table of Content:
Ice at a temperature of 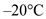 should be converted into water at a temperature of
should be converted into water at a temperature of 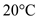 .
.
1 liter of water contains 100 g of mass.
Convert volume of 250 liters of water into mass of water in grams.
250 liters will have 250,000 g of water.
Ice increases in temperature from 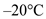 to
to 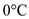 .
.
Use the specific heat of ice between temperatures of 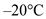 and
and 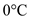 .
.
Specific heat of ice is the 0.465 calorie energy required for every 1 g of ice to be heated by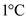 .
.
Find the calories  required to convert 250,000 g of water from
required to convert 250,000 g of water from 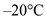 to
to 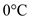 .
.

Ice undergoes a transformation from solid phase to liquid phase after reaching 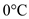 .
.
Use the latent heat of fusion (79.7 calories per gram of ice) to convert from solid phase to liquid phase.
Find the calories 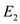 required to convert 250,000 g of ice to convert to liquid phase.
required to convert 250,000 g of ice to convert to liquid phase.

Water gets heated from 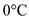 to
to 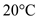 .
.
Specific heat of water is the 1 calorie of energy required for every 1 gram of substance to raise the temperature by 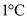 .
.
Find the calories 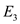 required to convert 250,000 g of water from
required to convert 250,000 g of water from 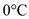 to
to 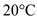 .
.

Compute the total energy required to be supplied ( ) while transforming ice to water
) while transforming ice to water

Amount of energy to be added to ice, to produce 250 liters of water, is 
People Also Search:
undamentals of hydraulic engineering systems
undamentals of hydraulic engineering systems 4th edition houghtalen
undamentals of hydraulic engineering systems 4th edition
undamentals of hydraulic engineering systems 4th edition download scribd
undamentals of hydraulic engineering systems 4th edition solution manual download pdf












